Triple-negative breast cancer (TNBC) doesn’t respond to hormone therapy or HER2-targeted drugs. That leaves chemotherapy as the main tool - but even that’s changing fast. Since 2020, new treatments have reshaped how doctors approach this aggressive form of breast cancer. Today, treatment isn’t one-size-fits-all. It’s guided by biomarkers, timing, and even personalized vaccines. If you or someone you know has been diagnosed with TNBC, understanding these shifts can make a real difference in outcomes.
Why TNBC Is Different
Most breast cancers grow because they feed on estrogen or progesterone, or because they overproduce the HER2 protein. Drugs exist to block those signals. TNBC doesn’t have any of them. That’s why it’s called ‘triple-negative’ - no receptors to target. It makes up about 10% to 15% of all breast cancers, but it’s responsible for a disproportionate number of deaths. It grows quickly, often spreads early, and has a higher chance of coming back within the first three to five years after treatment.
Because there were so few options, TNBC was treated like a blunt instrument - high-dose chemo, no exceptions. But now, researchers are learning that TNBC isn’t one disease. It’s a group of cancers with different biology. Some respond to immunotherapy. Others have BRCA mutations. A few show signs of immune activity. That’s why testing is no longer optional - it’s essential.
Standard Treatment Today: Chemo, Then What?
For early-stage TNBC, the standard starts with neoadjuvant chemotherapy - meaning chemo before surgery. The goal? Shrink the tumor so surgeons can remove it more easily, and see how well the cancer responds. A complete response - no cancer left in the breast or lymph nodes after chemo - means a much better chance of long-term survival.
The usual chemo combo includes anthracyclines like doxorubicin and taxanes like paclitaxel. But platinum drugs - cisplatin or carboplatin - are now often added, especially for younger patients or those with BRCA mutations. These drugs damage DNA in a way that’s especially effective against TNBC cells.
After chemo comes surgery, then often more chemo (adjuvant therapy). But here’s the big shift: we’re no longer just giving chemo and hoping. We’re layering in targeted treatments based on what the tumor tells us.
Immunotherapy: Turning the Body’s Defenses Against Cancer
For patients whose tumors express PD-L1 (about 40% of metastatic TNBC), adding immunotherapy to chemo has become standard. Pembrolizumab (Keytruda) and atezolizumab (Tecentriq) are checkpoint inhibitors that help the immune system recognize cancer cells as threats.
The KEYNOTE-522 trial showed that when pembrolizumab was added to chemo before surgery, 64.8% of PD-L1-positive patients had a pathologic complete response - meaning no detectable cancer after treatment. That’s compared to 44.1% with chemo alone. For those with PD-L1-negative tumors, the benefit was smaller, but still present.
But here’s something new: the UT Southwestern team found you don’t need to give pembrolizumab for months. Their 2025 trial gave just two doses before chemo, added radiation at the start, and still hit a 59% complete response rate. Toxicity dropped from 82% to 41%. That’s huge. Fewer side effects, same results. This could become the new model for early TNBC treatment.
PARP Inhibitors: Targeting BRCA Mutations
About 15% to 20% of TNBC patients carry a harmful BRCA1 or BRCA2 mutation - inherited changes that break the cell’s ability to repair DNA. PARP inhibitors like olaparib (Lynparza) and talazoparib (Talzenna) exploit this weakness.
In the OlympiAD trial, women with BRCA-mutated metastatic TNBC who took olaparib lived 7.8 months longer without their cancer worsening compared to those on standard chemo. Side effects included fatigue and low blood counts, but many patients tolerated it well. That’s why BRCA testing is now recommended for everyone diagnosed with TNBC - not just those with a family history.
Testing for broader DNA repair defects - called homologous recombination deficiency (HRD) - is also becoming common. Even patients without BRCA mutations but with HRD may benefit from PARP inhibitors. This is still being studied, but it’s expanding who can be helped.
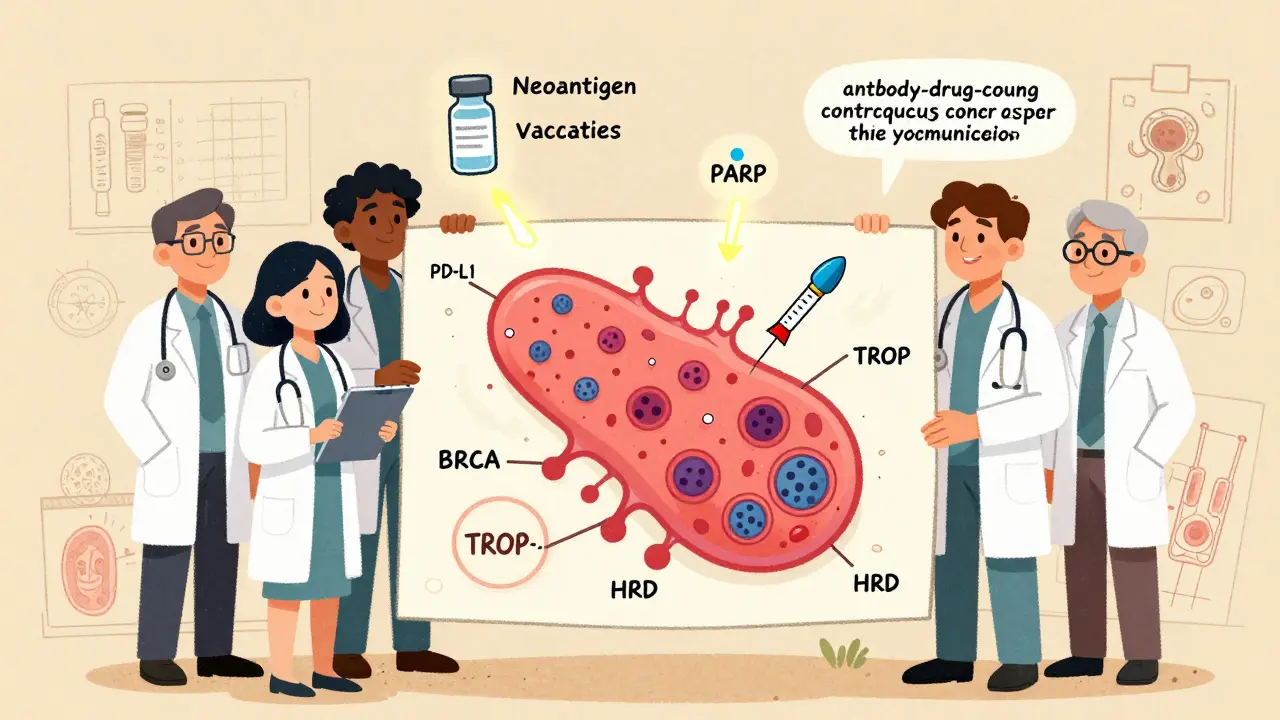
Antibody-Drug Conjugates: Smart Bombs for Cancer Cells
Sacituzumab govitecan (Trodelvy) is an antibody-drug conjugate - a missile that finds cancer cells and drops a toxic payload right on them. It targets TROP-2, a protein found on most TNBC cells.
In the ASCENT trial, women with metastatic TNBC who had already tried two types of chemo saw their cancer shrink in 35% of cases. Their median survival improved by nearly 5 months compared to standard chemo. The hazard ratio for death was 0.43 - meaning a 57% lower risk of dying.
But it’s not without risks. About 61% had severe low white blood cell counts. Diarrhea happened in 37% of cases. Still, for patients out of options, it’s a lifeline.
Another ADC, trastuzumab deruxtecan (Enhertu), was originally designed for HER2-positive cancers. But in the DESTINY-Breast04 trial, it worked in TNBC patients with even low levels of HER2 - a group previously thought untreatable. Response rates hit 37%. This is changing how we define HER2 - it’s not just positive or negative anymore.
Emerging Strategies: Dual Targeting and Personalized Vaccines
Researchers are now combining drugs to hit cancer from multiple angles. One promising approach pairs CDK12 inhibitors with PARP inhibitors. In lab models, this combo blocked tumor growth in 68% of cases - far better than either drug alone. Similar strategies use CDK4/6 and PI3K inhibitors together to shut down multiple growth signals at once.
But the most exciting development is coming from Houston Methodist Hospital. They’ve built a personalized neoantigen vaccine - made from a patient’s own tumor DNA. After sequencing the tumor, they create a custom vaccine in-house within six weeks. It’s designed to train the immune system to recognize unique mutations in that specific cancer.
In phase I trials, 78% of patients showed a strong immune response. When combined with pembrolizumab, the vaccine helped clear residual cancer cells after chemo. This isn’t just treatment - it’s prevention of recurrence. If it works in larger trials, it could change how we think about TNBC forever.
What’s on the Horizon? Trials to Watch
Over 1,500 clinical trials for TNBC are active worldwide. Here are a few to watch:
- Datopotamab deruxtecan - a new TROP-2 ADC from Roche, showing early promise in phase III trials.
- Adagloxad simolenin - a vaccine targeting a protein found in 90% of TNBC tumors.
- Durvalumab (Imfinzi) + chemo - the GeparNuevo trial showed a 92.5% three-year survival rate in early TNBC, beating chemo alone.
- CDK7/HDAC1 and CDK9/EZH2 inhibitors - targeting the cancer’s transcription machinery to stop it from making proteins it needs to survive.
These aren’t just lab experiments. Many are in late-stage trials and could be approved by 2027.
Testing Is Non-Negotiable
You can’t choose the right treatment without the right tests. At diagnosis, every TNBC patient should get:
- PD-L1 testing - using the 22C3 pharmDx assay - to see if immunotherapy is an option.
- BRCA1/2 germline testing - even if there’s no family history. About half of BRCA mutations in TNBC are new, not inherited.
- HRD testing - to identify those who might benefit from PARP inhibitors beyond BRCA carriers.
- Tumor mutational burden (TMB) - high TMB may predict better response to immunotherapy.
These aren’t luxury tests. They’re the foundation of modern care. Without them, you’re flying blind.
Challenges That Remain
Despite progress, TNBC is still deadly. Five-year survival for metastatic TNBC is only 12% to 15% - far lower than other breast cancer types. Many patients develop resistance. Tumors are smart. They change, adapt, and find new ways to survive.
Access is another problem. In low- and middle-income countries, only 35% to 40% of patients get the biomarker testing needed for these new therapies. Cost, infrastructure, and awareness gaps mean the breakthroughs aren’t reaching everyone.
And while treatments are improving, they’re still harsh. Fatigue, nerve damage, low blood counts, diarrhea - these side effects impact quality of life. The goal now isn’t just survival. It’s survival with dignity.
The Future Is Personalized
By 2028, experts predict over half of TNBC treatment decisions will be based on deep molecular profiling - not just one gene or one protein, but the whole picture: DNA, RNA, immune markers, tumor environment. We’re moving from ‘what type of cancer’ to ‘what is this cancer doing?’
That’s why the future isn’t just about more drugs. It’s about smarter sequencing - when to give radiation, how many doses of immunotherapy, which chemo to pair with which targeted agent. The UT Southwestern protocol proves that less can be more. Timing matters as much as the drugs themselves.
TNBC is still a tough enemy. But the tools we have today are far more precise than they were five years ago. And the pipeline? It’s fuller than ever. For patients, that means hope isn’t just a word - it’s a treatment plan.




JUNE OHM
January 4, 2026 AT 07:21 AMThey’re lying to us. 🤫 The ‘breakthroughs’? All funded by Big Pharma. They want you hooked on chemo and $200k vaccines so you never get well - just managed. PD-L1? BRCA? HRD? All just codes to sell more drugs. I’ve seen people die after ‘successful’ trials. The real cure? Fasting, cannabis oil, and detoxing your lymph system. But they’ll never tell you that. 🌿💉 #WakeUp
Philip Leth
January 4, 2026 AT 08:32 AMMan, I just lost my aunt to this. She got the whole nine yards - chemo, immunotherapy, the whole fancy pipeline. She said the worst part wasn’t the sickness… it was the feeling that doctors were treating a spreadsheet, not a person. Still, I’m glad they’re trying. Just wish they’d talk to patients like humans, not data points.
Shanahan Crowell
January 5, 2026 AT 15:10 PMTHIS IS HUGE!!! 🙌 We’re finally moving beyond ‘one-size-fits-all’ chemo bombs! The UT Southwestern two-dose immunotherapy protocol? Genius! Less toxicity, same results? That’s not progress - that’s a revolution! And personalized vaccines? That’s sci-fi becoming real! If this works at scale, we’re looking at a future where cancer isn’t a death sentence - it’s a manageable condition! Keep pushing, scientists!!!
Kerry Howarth
January 6, 2026 AT 09:12 AMTesting is non-negotiable. Period.
Joy F
January 7, 2026 AT 15:18 PMLet’s deconstruct this ‘personalized medicine’ fantasy. You’re telling me we’ve moved from ‘one chemo fits all’ to ‘six different chemo cocktails based on seven biomarkers’ - and we call that progress? It’s just complexity masquerading as innovation. The tumor mutational burden? The homologous recombination deficiency? These aren’t biological truths - they’re statistical artifacts created by overpriced sequencing machines. And don’t get me started on the ‘personalized vaccine’ - it’s a $500,000 placebo wrapped in CRISPR glitter. We’re not curing cancer. We’re monetizing hope.
Meanwhile, the real enemy - environmental toxins, endocrine disruptors, processed food, chronic stress - is ignored because it doesn’t come with a patent. The system doesn’t want you healed. It wants you perpetually testing, perpetually treated, perpetually indebted.
The real breakthrough? Stop treating cancer like a puzzle to be solved with more drugs. Start treating it like a symptom of a broken society. But that’s too radical, isn’t it? Too… political.
Haley Parizo
January 7, 2026 AT 18:00 PMThey call this progress? You’ve got people in rural Alabama waiting six months for a BRCA test while billionaires get bespoke vaccines. This isn’t medicine - it’s a caste system with IV drips. The science is brilliant, yes. But the delivery? A joke. If your survival depends on your zip code, your insurance, and your ability to navigate a labyrinth of clinical trial portals, then we haven’t cured cancer - we’ve just made it a luxury good. And that’s not just unethical. It’s a crime.
Ian Detrick
January 7, 2026 AT 18:37 PMWhat’s wild is how fast this field moved. Five years ago, TNBC was a dead end. Now we’ve got ADCs, vaccines, combo therapies - and we’re learning that less chemo can sometimes be more effective. It’s not magic. It’s science, slowly, stubbornly, improving. I’m not naive - side effects are brutal, access is unequal, and costs are insane. But for the first time, there’s a real path forward. Not perfect. Not fair. But real. And that’s worth fighting for.
Brittany Wallace
January 8, 2026 AT 15:34 PMThank you for writing this. My sister just finished her last round of Trodelvy. The diarrhea was hell… but she’s NED. 🤍 I keep thinking about that Houston vaccine trial - if it works, it could mean no more fear of recurrence. I know it’s early, but I’m holding onto it. To everyone going through this: you’re not alone. And the science? It’s catching up to us. Slowly, but it’s catching up. 💪