When you pick up a prescription, you might not think twice about whether it’s the brand name or the generic version. After all, the FDA says they’re the same. But if you’ve been on a medication for years - say, for high blood pressure, epilepsy, or thyroid disease - you might have noticed something off. A weird side effect. A sudden spike in symptoms. Or maybe, nothing at all. The truth is, the long-term safety of generic drugs compared to brand-name versions isn’t as simple as it sounds.
What Does ‘Therapeutically Equivalent’ Really Mean?
The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. That’s the law. But here’s the catch: bioequivalence doesn’t mean identical. It means the generic must deliver the drug into your bloodstream within 80% to 125% of the brand’s rate. That’s a 45% range. For most drugs, that’s fine. For others? It’s a different story. Take levothyroxine, the drug used to treat hypothyroidism. A 2017 study found that patients switching from Synthroid to generic versions had a 12.3% higher chance of abnormal thyroid-stimulating hormone (TSH) levels. That might sound small, but for someone relying on precise hormone control, even a 10% fluctuation can mean fatigue, weight gain, or worse. The FDA allows this variation because it works for most people. But for those with narrow therapeutic index drugs - where the difference between a safe dose and a toxic one is tiny - that margin matters.Real-World Data: Generics Can Be Safer
One of the most surprising findings came from a 2020 study in Austria, tracking over 1.5 million people with chronic conditions between 2007 and 2012. Researchers looked at antihypertensive drugs - the kind people take for life. What they found flipped the script. Branded versions were linked to 53.8 deaths per 1,000 patient-years. Generics? Just 30.2. That’s nearly half the mortality rate. Major cardiac events like heart attacks and strokes were 39% lower in the generic group. The study didn’t just compare prescriptions - it used advanced statistical methods to control for age, income, prior illness, and even how often people filled their meds. The result? Generics weren’t just as safe. They were safer. Why? One theory: brand-name drugs often come with higher prices, which can lead to patients skipping doses or not refilling prescriptions. Generics are cheaper. People take them consistently. Better adherence means better outcomes. That’s not a drug difference. That’s a system difference.The Flip Side: When Switching Causes Problems
But here’s where it gets messy. There are documented cases where switching from brand to generic triggered real harm. One patient on generic ciprofloxacin kept getting infections. Their fever didn’t break. When they switched back to the brand, Ciproxin, symptoms vanished in days. Another person on generic levofloxacin had worsening fever until they went back to Tavanic. In both cases, the active ingredient was identical. So what changed? Inactive ingredients. Fillers. Coatings. Binders. These don’t affect the drug’s strength, but they can affect how it dissolves in your gut - or whether your body tolerates it. A person with a sensitive stomach might react to a dye in one generic version but not another. Or a coating that delays release might cause the drug to absorb too slowly, making it ineffective. A 2013 review found that 30% of patients improved after switching from generic to brand, 30% saw no change, and 30% got worse. That’s not random noise. That’s real, repeatable clinical variation.
It’s Not Brand vs Generic - It’s Manufacturer vs Manufacturer
Here’s something most people don’t know: many brand-name drugs are made by the same companies that make generics. These are called authorized generics. They’re chemically identical to the brand, just sold under a generic label. A 2018 study compared adverse event reports for amlodipine (a blood pressure drug). Brand-name reports made up 29.5% of the total. Authorized generics? 14.3%. Regular generics? 56.2%. That’s a huge gap. But when they compared authorized generics to regular generics, the difference vanished. The same drug, same manufacturer, same formula - just a different label. Same thing happened with losartan. The brand had more reports - but so did the generic made by a different company. The pattern wasn’t brand vs generic. It was manufacturer A vs manufacturer B. This suggests that many safety concerns aren’t about the generic label at all. They’re about inconsistent quality control across different factories.Where the Drugs Come From Matters
A 2018 study from Ohio State University looked at FDA adverse event reports and found a startling trend. Generic drugs made in India had a 54% higher rate of severe adverse events - including hospitalizations and deaths - compared to those made in the U.S. The difference was most pronounced in older, well-established drugs like ciprofloxacin. Indian-made versions showed a 62% higher rate of hospitalizations due to severe reactions. The researchers controlled for dosage, patient age, and prescription volume. The gap stayed. Why? Some Indian manufacturers cut corners. They use cheaper raw materials. They skip stability tests. They don’t maintain clean rooms. The FDA inspects foreign plants, but not enough. In 2022, nearly 80% of generic drugs sold in the U.S. were made overseas. Most of them came from India and China. That doesn’t mean all Indian generics are dangerous. Many are fine. But the risk isn’t zero. And patients have no way of knowing which batch came from which country.What the FDA Says - and Doesn’t Say
The FDA insists generics are safe. And for most people, they are. The agency points to over 2,000 bioequivalence studies showing generics perform within a narrow range of brand drugs. They also cite a 2021 Harvard study of 136,000 older adults on blood pressure meds. After generics hit the market, there was no spike in ER visits or hospitalizations. But here’s the problem: those studies look at short-term outcomes. They track hospitalizations. They don’t track subtle declines - fatigue, brain fog, mood swings, slow kidney function - that might build up over years. They don’t follow patients for decades. And they don’t test how different formulations interact with other drugs in complex regimens. The FDA’s current system was designed in the 1980s. It’s built for simple pills. It’s not built for complex delivery systems like inhalers, long-acting injectables, or topical creams. In 2022, the FDA admitted as much and released new guidance for testing these “complex generics.” But enforcement? Still patchy.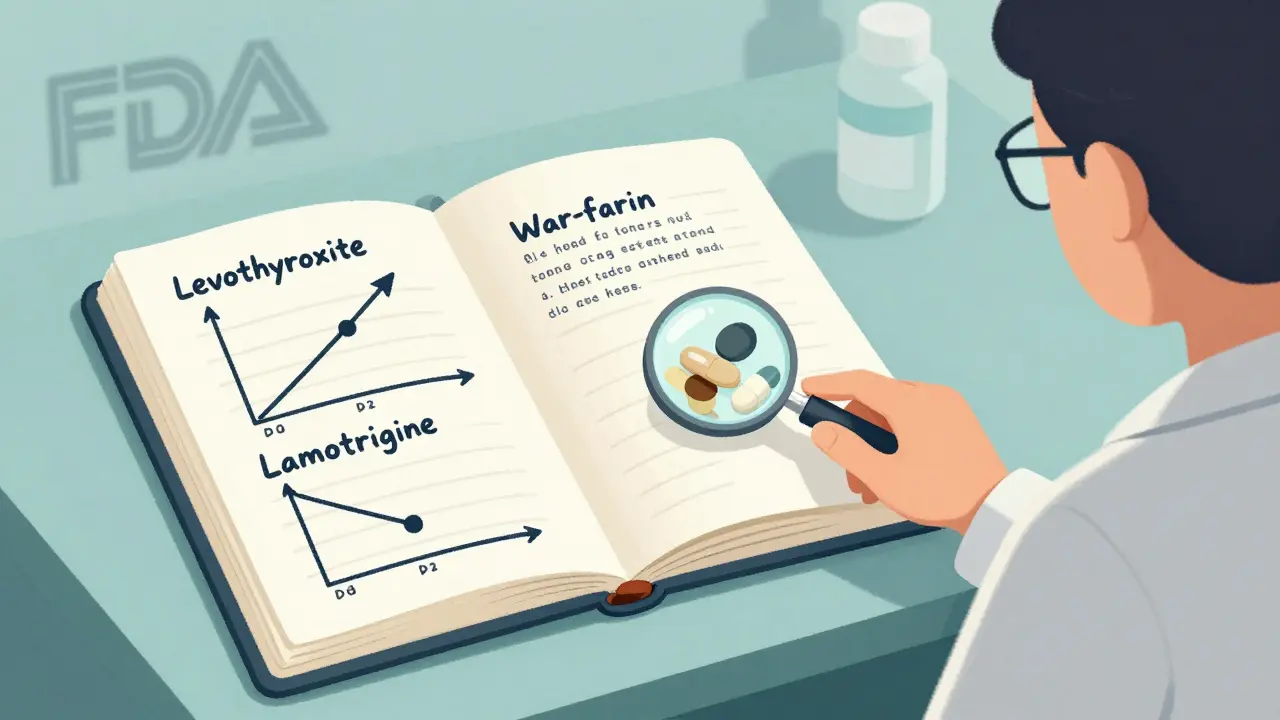
Who Should Be Careful?
Not everyone needs to worry. If you’re taking a generic for allergies, acid reflux, or antibiotics, you’re probably fine. But for certain drugs, the stakes are higher:- Levothyroxine - Even small changes in absorption can throw off thyroid levels.
- Warfarin - A 10% difference in blood levels can mean a clot or a bleed.
- Lamotrigine - Used for epilepsy and bipolar disorder. One Reddit user reported their seizures jumped from 1-2 per month to 8-10 after switching to generic.
- Cyclosporine - Used after transplants. Tiny changes can lead to organ rejection.





Josh Potter
December 17, 2025 AT 00:54 AMBro. I switched my levothyroxine to generic and started feeling like a zombie. No energy, gained 15 lbs, my brain felt like it was wrapped in saran wrap. I went back to Synthroid and boom - I’m human again. FDA can say what they want, but my body knows the difference.
Evelyn Vélez Mejía
December 18, 2025 AT 15:31 PMThe pharmacological equivalence paradigm is a reductive illusion, predicated upon the assumption that molecular identity equates to physiological harmony. Yet, the human organism is not a beaker in a lab - it is a symphony of epigenetic, metabolic, and psychological variables. The 80–125% bioequivalence window is not a margin of error; it is a chasm through which individual sovereignty dissolves into the bureaucratic anonymity of cost-efficiency.
When we reduce a life-sustaining medication to a commodity, we do not merely economize - we erode the sacred covenant between healer and healed.
Meghan O'Shaughnessy
December 19, 2025 AT 22:51 PMI’m a nurse in a rural clinic. I’ve seen this firsthand. Elderly patients on warfarin - one week on generic, next week INR skyrockets. They don’t know why. No one tells them the manufacturer changed. We have to play detective just to keep them alive. The system is broken.
Kaylee Esdale
December 19, 2025 AT 23:08 PMMy mom takes lamotrigine. Switched generics and she got dizzy, started crying for no reason. We switched back. Done. No drama. If it ain’t broke, don’t fix it. Your meds are not a gamble. Just ask your doc to write ‘do not substitute’ if you feel off.
Raven C
December 21, 2025 AT 07:59 AMIt is, frankly, astonishing that any rational adult would consider substituting a branded pharmaceutical with a generic - especially when one considers the abysmal quality control standards in foreign manufacturing facilities, the lack of post-marketing surveillance, and the fact that many generics are produced in factories that have been cited for data falsification by the FDA. This is not medicine. This is pharmaceutical roulette.
And yet, we are told to be ‘grateful’ for the cost savings? Grateful for the risk? Grateful for the potential for iatrogenic harm? One does not save money on one’s life.
Jessica Salgado
December 22, 2025 AT 04:55 AMI had a friend who took a generic version of cyclosporine after a kidney transplant. She got sick. Really sick. Turned out the batch was made in India and the dissolution rate was off. She almost lost the kidney. She’s fine now - back on the brand. But imagine if no one had caught it. This isn’t about money. It’s about trust.
And why can’t we just scan a QR code on the bottle to see where it was made? Why is that too much to ask?
Brooks Beveridge
December 22, 2025 AT 05:11 AMHey everyone - I get it. Some generics are sketchy. But don’t throw the baby out with the bathwater. I’ve been on generic amlodipine for 8 years. Zero issues. My BP is perfect. My pharmacist knows which manufacturers are solid. Ask them. They’re your allies. And yes - some Indian makers are sketchy, but so are some U.S. ones. It’s about the factory, not the label. Stay informed, don’t panic.
And if you feel weird after a switch? Speak up. Report it to MedWatch. That’s how we fix this.
Anu radha
December 24, 2025 AT 04:24 AMI am from India. Many of us make these medicines. We work hard. Not all factories cut corners. Some are very good. Please do not judge all of us because of a few bad ones. We want to help people too.
Salome Perez
December 26, 2025 AT 01:57 AMThere’s a critical distinction here that’s being overlooked: authorized generics. They’re chemically identical to the brand, often made in the same facility, just sold under a different label - and they’re usually priced like generics. If you’re concerned about quality but need affordability, ask your pharmacist for the authorized generic. It’s the best of both worlds. And yes - the manufacturer matters more than the brand name. Always ask: ‘Who made this?’
Kent Peterson
December 26, 2025 AT 02:43 AMOh, here we go again - the ‘generic is dangerous’ fear-mongering. Let’s not forget: 92% of prescriptions are generic. If they were killing people, we’d be in a national crisis. The FDA has approved over 2,000 bioequivalence studies. The Austrian study you cited? It showed generics had LOWER mortality. So why are you cherry-picking outliers? Because it feels good to be afraid? Because you think you’re ‘in the know’? Wake up. This isn’t about science - it’s about anti-generic propaganda funded by Big Pharma.