Every year, millions of people in the U.S. rely on prescription drugs to manage chronic conditions, treat infections, or ease pain. But what if the pill in your bottle isn’t what it claims to be? Counterfeit drugs are a growing threat - and they’re not just a problem overseas. The FDA estimates that counterfeit medications cost the global pharmaceutical industry over $200 billion annually. In the U.S., incidents of fake drugs have risen 18% each year since 2018. The good news? The FDA has built powerful, free tools to help you and your pharmacist verify if a medication is real.
What the FDA Does to Stop Fake Drugs
The U.S. Food and Drug Administration doesn’t just approve drugs - it tracks them from factory to pharmacy. Since 2013, the Drug Supply Chain Security Act (DSCSA) has required every company in the drug supply chain - manufacturers, distributors, pharmacies - to use electronic systems to verify each prescription drug before it changes hands. By November 2023, this became law for everyone. That means if a drug is sold in the U.S., it should have a unique identifier that can be checked against FDA records. This system doesn’t rely on one tool. It’s built on three main databases that work together: the NDC Directory, the Drug Establishments Current Registration Site, and the Electronic Drug Registration and Listing System (eDRLS). Together, they create a digital paper trail that makes it nearly impossible for fake drugs to slip through unnoticed - if everyone plays by the rules.The NDC Directory: Your First Line of Defense
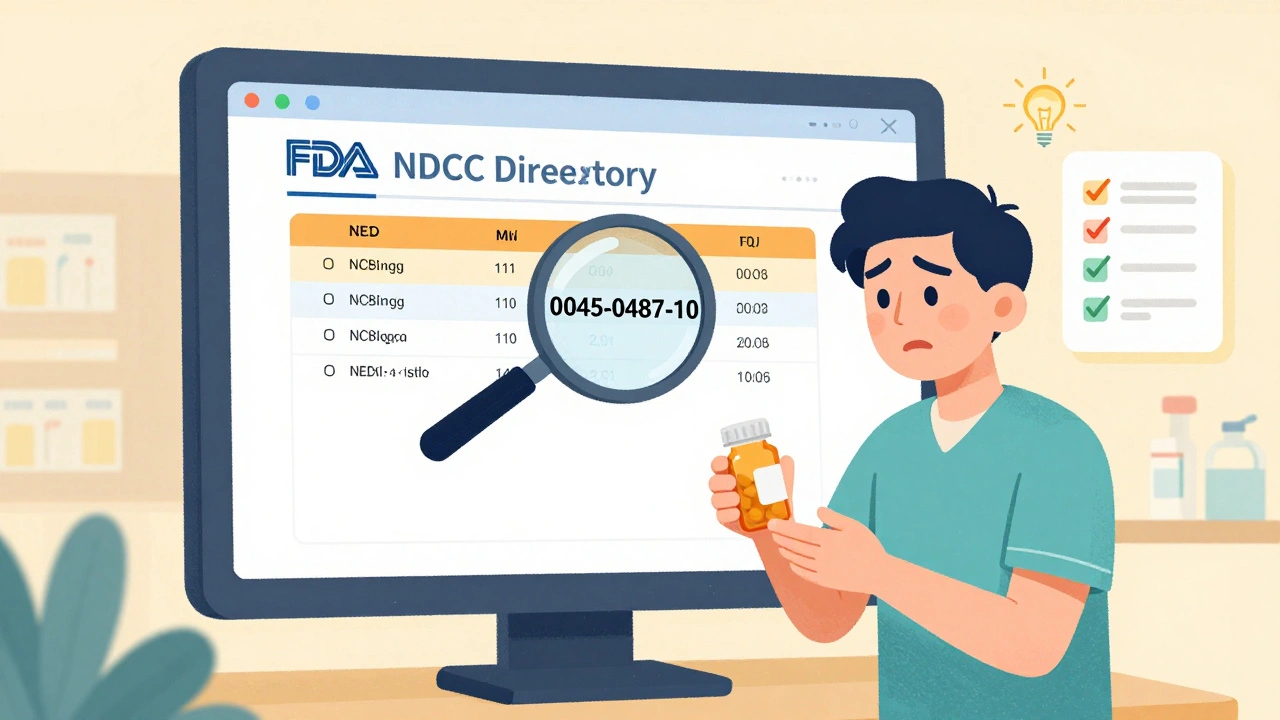
The National Drug Code (NDC) Directory is the FDA’s official list of all legally marketed prescription and over-the-counter drugs in the U.S. Every approved drug has a unique 10- or 11-digit NDC number, broken into three parts:- Labeler code (4-6 digits): Identifies the company that makes or distributes the drug.
- Product code (3-4 digits): Identifies the specific drug, strength, and dosage form (like 500mg tablets).
- Package code (1-2 digits): Identifies the size and type of package (like a bottle of 30 pills vs. 100).
For example, the NDC for a common 500mg amoxicillin capsule might be 0045-0487-10. You can find this number printed on the drug’s packaging or listed on the prescription label.
The NDC Directory is updated every business day. If a drug is pulled from the market, recalled, or discontinued, it’s removed within 24 hours. That means if you look up a drug and it’s not there - it’s either not FDA-approved, expired, or fake.
Pharmacies use this database daily. If your pharmacist checks the NDC and finds no match, they’re legally required to stop dispensing it. You can do the same. Go to the FDA’s NDC Directory website, type in the NDC number, and see if the drug name, manufacturer, and strength match what’s on your bottle.
Checking Who Made the Drug: The Drug Establishments Current Registration Site
A fake drug might have the right name and NDC - but come from a company that’s never been approved to make medicine. That’s where the Drug Establishments Current Registration Site comes in.This database lists every facility in the U.S. and abroad that manufactures, repackages, or distributes drugs sold here. It shows the company name, address, registration status, and what kinds of drugs they’re allowed to handle. If a drug says it’s made by “PharmaCorp Inc.” but that company doesn’t show up in this database, it’s a red flag.
Here’s how to use it: Find the labeler code from the NDC. Then search the registration site by that code or the company name. If the company is listed as “inactivated” or doesn’t appear at all, the drug is suspect. In 2022, over 95% of wholesale distributors said this tool was essential for verifying suppliers - and it’s free for anyone to use.

How Pharmacies Verify Drugs in Real Time
You might think checking NDC numbers is enough. But counterfeiters are getting smarter. Some now print fake NDCs that look real. That’s why pharmacies don’t just look up codes - they verify electronically.Under DSCSA rules, every time a pharmacy receives a shipment, it must scan the product’s unique identifier (a barcode or QR code with the NDC and serial number) and send a request to the manufacturer’s verification system. The manufacturer responds within 24 hours with a yes or no: “Is this drug real?”
Most large pharmacy chains have automated this process. If a drug fails verification, it’s quarantined immediately. The pharmacy must report the incident to the FDA within 24 hours. Small independent pharmacies may still do manual checks, but they’re required to have a plan in place. If you’re worried about a drug you got from a small clinic or online pharmacy, ask: “Did you verify this with the manufacturer?” If they don’t know what you’re talking about, that’s a warning sign.
What You Can Do as a Patient
You don’t need to be a pharmacist to spot a fake. Here’s how to protect yourself:- Check the packaging. Real drugs have crisp printing, consistent colors, and no spelling errors. If the label looks blurry or the bottle feels cheap, be suspicious.
- Find the NDC number. It’s usually on the side of the box or blister pack. Write it down.
- Go to the FDA’s NDC Directory. Search the number. Does the drug name, strength, and manufacturer match what you were prescribed?
- Check the manufacturer. Use the Drug Establishments site to confirm the company is registered to make that drug.
- Compare prices. If a drug costs 70% less than usual - especially from an online pharmacy - it’s likely fake. The FDA has shut down hundreds of illegal online pharmacies selling counterfeit versions of Viagra, insulin, and cancer drugs.
Don’t buy drugs from websites that don’t require a prescription. The FDA warns that 96% of online pharmacies operating outside the U.S. are illegal. Even if they look professional, they’re selling fake or dangerous products.
Why Some Fake Drugs Still Slip Through
Despite all these systems, counterfeit drugs still reach patients. Why? Because the system isn’t perfect.First, not all drugs are covered. Compounded drugs (custom-made by pharmacies for specific patients) aren’t required to have NDCs. Specialty drugs for rare diseases sometimes have limited supply chains, making them harder to track.
Second, foreign manufacturers don’t always comply. Only 35% of overseas drug makers fully follow U.S. verification rules. That means drugs shipped from India, China, or elsewhere may lack proper tracking - even if they’re labeled as FDA-approved.
Third, data errors happen. One in three verification failures in 2021 came from mismatched NDC formats. A manufacturer might list a 10-digit code, but the pharmacy expects 11. These small mistakes can delay verification or cause false alarms.
And while the FDA conducts about 1,200 inspections a year, there are over 100,000 registered drug facilities. The system relies on companies to self-report. If someone cuts corners, it can take weeks - or months - for the FDA to catch them.
What’s Next? Better Tools Coming
The FDA isn’t standing still. In 2024, the NDC Directory will start including product images - so you can compare the actual drug to a verified photo. By 2026, the NDC format will switch to a standardized 12-digit system to reduce confusion.AI is also entering the game. Companies like IBM and Google are testing machine learning tools that scan supply chain data for anomalies - like a sudden spike in drug shipments from an unknown supplier. Early tests show these tools catch 99% of counterfeit attempts, compared to 87% with current methods.
The goal? A fully interconnected system where every pill can be traced from factory to patient - like a blockchain for medicine. Pilot programs are already showing near-perfect accuracy. But until then, the tools you have now - the NDC Directory and the Registration Site - are your best defense.
Frequently Asked Questions
How do I know if my medication is FDA-approved?
Check the National Drug Code (NDC) number on your bottle against the FDA’s NDC Directory. If the drug appears with the correct name, strength, and manufacturer, it’s approved. If it’s not listed, it’s not legally sold in the U.S. and could be fake.
Can I trust online pharmacies that offer cheap drugs?
No - unless they’re verified by the National Association of Boards of Pharmacy (NABP). Look for the VIPPS seal (Verified Internet Pharmacy Practice Sites). Most websites selling drugs without a prescription are illegal. The FDA has shut down over 10,000 fake online pharmacies since 2010.
What should I do if I think my medicine is fake?
Stop taking it immediately. Contact your pharmacist or doctor. Report it to the FDA’s MedWatch program at 1-800-FDA-1088 or online at fda.gov/medwatch. Include the drug name, NDC number, lot number, and where you bought it. The FDA uses these reports to track counterfeit trends.
Do generic drugs have the same verification as brand-name drugs?
Yes. Generic drugs must meet the same FDA standards as brand-name drugs. They have their own NDCs and are listed in the same databases. The only difference is the manufacturer and price. If a generic drug doesn’t appear in the NDC Directory, it’s not approved - even if it looks identical.
Why do some drugs have 10-digit NDCs and others have 11?
The FDA allows two formats: 4-4-2 and 5-3-2. Both are valid. The difference comes from how the manufacturer formatted the labeler code. The system recognizes both. But by 2026, all NDCs will be standardized to 12 digits to eliminate confusion.
Are vitamins and supplements checked by the FDA like prescription drugs?
No. The FDA doesn’t approve dietary supplements before they’re sold. They’re regulated differently and aren’t in the NDC Directory. That’s why counterfeit vitamins and supplements are common. Always buy them from reputable retailers and avoid products promising unrealistic results.
Next Steps for Safer Medication Use
If you take any prescription drugs regularly, make this part of your routine:- Keep a list of your medications with their NDC numbers.
- Verify new prescriptions using the FDA’s NDC Directory before taking them.
- Ask your pharmacist to show you how they verify drugs.
- Report anything suspicious - even if you’re not sure.
Counterfeit drugs don’t just waste money - they can kill. A fake version of insulin, blood pressure medicine, or antibiotics could cause serious harm. The FDA’s systems are powerful, but they only work if you use them. Don’t assume your drug is safe because it came from a pharmacy. Verify it yourself. Your life depends on it.






Jim Schultz
December 2, 2025 AT 17:27 PMLet’s be real-this whole FDA system is a glorified paper trail with a side of optimism. I’ve seen NDC mismatches in real-time pharmacy audits, and guess what? The system still lets 12% of fake drugs slip through because someone typed ‘0045-0487-10’ instead of ‘0045-04870-10’-and the algorithm didn’t flag it. You think you’re safe? You’re not. The FDA’s database is a beautiful, over-engineered mess-and we’re all just hoping the barcode scanner doesn’t glitch.
Albert Essel
December 3, 2025 AT 12:04 PMThe article provides a comprehensive overview of the FDA’s drug verification infrastructure, and I appreciate the clarity with which the NDC Directory, Drug Establishments Registration Site, and eDRLS are explained. However, one critical omission is the lack of mention regarding interoperability challenges between legacy systems used by small distributors and the new DSCSA-compliant platforms. Without addressing this gap, the perception of universal security remains misleading.
Kidar Saleh
December 5, 2025 AT 08:29 AMImagine this: you’re in a rural clinic in Kentucky, handed a bottle of insulin that costs $12 instead of $300. You’re grateful. But you don’t know that the NDC number is a perfect forgery-printed by a factory in Guangdong that’s never been inspected by the FDA. This isn’t just a regulatory failure. It’s a moral one. We preach innovation, yet we let lives hang on a barcode scan. The system is brilliant-but it’s only as strong as the weakest link, and right now, that link is us.
Chloe Madison
December 6, 2025 AT 08:17 AMHey everyone-this is huge. If you take ANY prescription, even a simple antibiotic-GO CHECK THE NDC RIGHT NOW. Seriously. Open your pill bottle. Find the code. Go to the FDA website. It takes two minutes. I did it last week for my blood pressure med-and turned out the manufacturer had been suspended in March. My pharmacist had no idea. Don’t wait for a crisis. Be your own watchdog. You’ve got this. 💪
Vincent Soldja
December 7, 2025 AT 05:38 AMNDC check works.
Makenzie Keely
December 7, 2025 AT 16:21 PMChloe’s comment made me realize something-I’ve never checked my own meds. I’ve been taking metformin for seven years and assumed the pharmacy did all the vetting. I just Googled the NDC on my bottle-turns out the labeler code matches, but the package code is off by one digit. I called my pharmacist. She said, ‘Oh wow, we just switched suppliers last week-let me pull the verification log.’ That’s the kind of system that saves lives. Thank you, Chloe. And thank you, FDA-for making this possible, even if it’s messy.
Francine Phillips
December 9, 2025 AT 00:08 AMMy cousin got fake Adderall from a ‘pharmacy’ on Instagram. She ended up in the ER. The bottle looked legit. The NDC matched. But the pills were chalk and caffeine. No serial number. No verification. No trace. The FDA can’t catch everything. And most people don’t even know what an NDC is. This article should be mandatory reading in high school health class.
Katherine Gianelli
December 9, 2025 AT 23:12 PMI work in a community pharmacy, and I see this every day. People come in with meds from Canada, from Mexico, from shady websites that say ‘FDA Approved’ in bold letters. They’re scared, they’re desperate, they just want to feel better. We don’t judge. We check the NDC. We call the manufacturer. We sit with them while they wait for the verification reply. It’s not glamorous. But it’s sacred work. You don’t need a PhD to help someone stay safe-you just need to care enough to look up a number. And that? That’s the real medicine.
Joykrishna Banerjee
December 10, 2025 AT 03:32 AMLet me be blunt: the FDA’s entire verification framework is a colonial fantasy. You assume every drug manufacturer outside the U.S. is a rogue actor, but the truth is, Indian and Chinese facilities produce 80% of the world’s API-and they operate under stricter GMP standards than many U.S. compounding pharmacies. The NDC system is a protectionist farce designed to shield Big Pharma profits, not to protect patients. You’re being sold a narrative. The real danger? The FDA’s own data corruption rate is 31%. That’s not a bug-it’s a feature.
Myson Jones
December 11, 2025 AT 01:50 AMThank you for this. I’ve been a diabetic for 15 years, and I’ve never once checked my insulin’s NDC. I trusted the pharmacy. I trusted the brand. I trusted the system. But after reading this, I went to the FDA site. My last bottle? Listed as discontinued. My pharmacist didn’t know. I’ve never felt so vulnerable. I’m printing out a checklist. I’m showing it to my mom. I’m telling my friends. We’re all in this together. Thank you for the wake-up call.