Every year, millions of prescription drugs move through a complex network of manufacturers, wholesalers, and pharmacies before reaching your medicine cabinet. But somewhere in that chain, a fake pill could slip in - a counterfeit drug that looks real but contains the wrong ingredients, too much active drug, or even toxic substances. The DSCSA track-and-trace system was built to stop that before it happens.
What the DSCSA Actually Does
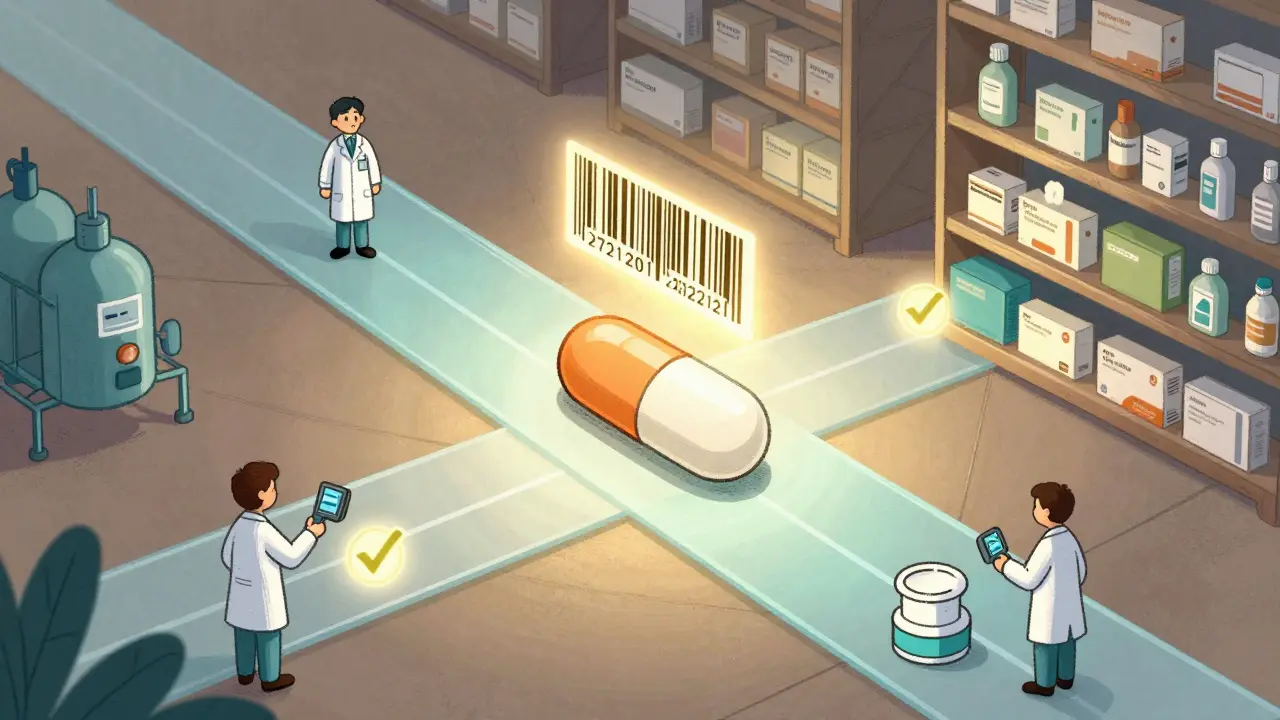
The Drug Supply Chain Security Act (DSCSA) isn’t just another regulation. It’s the first federal law in the U.S. that forces every player in the drug supply chain - from makers to pharmacies - to track each pill, capsule, or vial with a unique digital fingerprint. Think of it like a serial number on a smartphone, but for medicine. Since November 2024, every package of prescription drug must have a 20-character serial number, lot number, expiration date, and the National Drug Code (NDC) - all in a barcode that machines can read and systems can verify.This wasn’t always the case. Before DSCSA, drug tracking was a mess. Some states had their own rules. Others had none. Counterfeiters exploited those gaps. A fake version of a blood pressure pill could be shipped from a warehouse in Texas to a small pharmacy in Ohio without anyone knowing it wasn’t real. DSCSA changed that by creating one nationwide standard. No more patchwork. No more loopholes.
How the System Works in Real Time
When a manufacturer ships a box of insulin, each bottle gets a unique serial number. That number, along with the lot and expiration date, gets recorded in an electronic system. When the wholesaler receives it, they scan the barcode. Their system checks if the serial number matches what the manufacturer sent. If it doesn’t - if the number was copied, stolen, or made up - the system flags it immediately.This happens again at every step: when the drug moves to a distributor, then to a hospital or pharmacy. At each point, the system asks: Is this package legitimate? The answer must come back in seconds. By law, every dispenser - that’s your local pharmacy - must be able to verify a drug’s authenticity within 24 hours of receiving it.
It’s not just about scanning barcodes. The system also requires three electronic documents with every shipment:
- Transaction Information (TI): What drug is this? What’s the lot? When does it expire?
- Transaction History (TH): Who handled this drug before? Every stop on its journey.
- Transaction Statement (TS): A legal certification that the drug wasn’t stolen or tampered with.
If any of these don’t match up - if the serial number doesn’t line up with the lot, or the history shows a stop that never happened - the pharmacy can’t accept the shipment. The drug gets quarantined. An investigation starts. And the FDA is notified.
Why This Cuts Counterfeit Drugs by 95%
Counterfeit drugs don’t usually come from overseas labs anymore. They come from inside the system - stolen packages, diverted inventory, or fake documentation. DSCSA makes that nearly impossible.Before DSCSA, if a thief stole a box of 100 pills, they could resell them as new. No one could prove they were stolen. Now, each pill has a unique serial number. If a pharmacy gets a box with serial numbers that don’t match the manufacturer’s database, the system knows it’s fake. The FDA estimates this system reduces counterfeit drugs entering the supply chain by 95%.
It also makes recalls faster and smarter. Before DSCSA, if one bad batch of pills was found, companies had to recall entire product lines - thousands of boxes, even if only one lot was contaminated. Now, they can pinpoint the exact lot, the exact pallet, even the exact pharmacy that received it. That saves lives and money.

The Real Cost of Compliance
This system didn’t come cheap. For big manufacturers and distributors, it was a multi-million-dollar overhaul. But the real pain has been for small pharmacies.A 2023 survey by the National Community Pharmacists Association found that 68% of independent pharmacies said DSCSA compliance was their biggest technology challenge. The average cost to upgrade systems? Around $185,000. That’s more than most small pharmacies make in profit in a year. Some had to delay hiring staff. Others took out loans. Walgreens spent $120 million just on DSCSA tech upgrades between 2021 and 2022.
But the cost of not doing it? Higher. A single incident of a counterfeit drug reaching a patient can lead to lawsuits, lost trust, and even death. The FDA issued a warning letter to a regional distributor in 2022 for failing to investigate suspect products - a clear violation. That’s the kind of risk no pharmacy can afford.
Where the System Still Stumbles
DSCSA works - but it’s not perfect. The biggest problem? Data mismatches.Imagine two pharmacies using different software. One system records a serial number as “ABC123XYZ,” the other as “abc123xyz.” Even though it’s the same number, the system sees it as a mismatch. That’s not rare. A 2022 survey found 42% of companies struggled with data errors between trading partners.
Another issue? EPCIS - the global standard for sharing tracking data - isn’t used the same way by everyone. Some vendors interpret it loosely. Others stick to the rules. That creates delays. Pharmacists on Reddit have reported 2- to 3-day holds on shipments because systems couldn’t verify the data. That slows down care. It frustrates staff. It costs money.
And while 98% of manufacturers and 95% of wholesalers are compliant, only 72% of pharmacies are fully ready. Chain pharmacies like CVS and McKesson hit compliance early. They had the resources. Independent pharmacies are still catching up.

What Happens Now? The Stabilization Period
The November 27, 2024 deadline wasn’t the end - it was the start of the real test. The FDA gave the industry a one-year stabilization period to fix glitches without penalties. That ends in November 2025.Now, enforcement is tightening. The FDA expects every system to work as designed. No more excuses. If a pharmacy can’t verify a drug within 24 hours, they’re in violation. If a distributor ships without full transaction data, they’re breaking the law.
And the system is evolving. In March 2023, FDA Commissioner Dr. Robert Califf said the agency is looking at extending DSCSA to high-risk over-the-counter drugs - things like insulin pens, asthma inhalers, or erectile dysfunction pills. These aren’t prescription-only, but they’re still targeted by counterfeiters.
What You Should Know as a Patient
You don’t need to scan barcodes or understand EPCIS. But you should know this: the system is working. If you get a prescription, chances are it’s been tracked from the factory to your hands. No one can slip a fake pill into that chain without getting caught.If your pharmacy ever says a drug can’t be verified - don’t take it. Walk out. Call the FDA’s MedWatch hotline. That’s what the system is designed for: to give you a safety net. You don’t have to be an expert. You just have to trust that the rules are in place - and that they’re being enforced.
What’s Next?
The DSCSA track-and-trace system isn’t just about stopping fakes. It’s about building trust. In a world where drug prices are rising and supply chains are fragile, knowing your medicine is real is worth more than money.By 2027, PwC projects DSCSA will save $2.3 billion a year - through fewer recalls, less drug diversion, and lower liability costs. That money goes back into patient care. Into research. Into keeping the system running.
The technology is here. The rules are clear. The deadline passed. Now, it’s about making sure every pharmacy, every distributor, every manufacturer stays on track. Because when it comes to your health, there’s no room for error.
What is the DSCSA and why does it matter?
The Drug Supply Chain Security Act (DSCSA) is a federal law that requires every prescription drug in the U.S. to have a unique serial number and be tracked electronically from manufacturer to pharmacy. It matters because it stops counterfeit, stolen, or contaminated drugs from reaching patients. Before DSCSA, fake pills could slip through the system unnoticed. Now, every package can be verified in seconds.
How does DSCSA stop counterfeit drugs?
Each drug package gets a unique 20-character serial number tied to its lot and expiration date. When the drug moves through the supply chain - from maker to wholesaler to pharmacy - every step must be verified electronically. If a serial number doesn’t match the manufacturer’s database, the system flags it as suspect. The drug is quarantined, investigated, and reported to the FDA. Counterfeiters can’t copy these numbers without being caught.
Do all pharmacies have to comply with DSCSA?
Yes. All dispensers - including retail pharmacies, hospitals, and clinics - must be able to verify the authenticity of any prescription drug they receive. By November 2024, full compliance was required. While most chain pharmacies met the deadline, about 28% of independent pharmacies were still working on compliance as of mid-2023. The FDA is now enforcing these rules strictly.
What happens if a pharmacy gets a suspect drug?
If a drug is flagged as suspect - meaning its serial number doesn’t match, or the transaction history is incomplete - the pharmacy must immediately quarantine it. Then they must investigate: check the serial number against the manufacturer’s database, look for signs of tampering, and determine if it’s counterfeit, stolen, or illegitimate. If confirmed, they must notify the manufacturer and the FDA within 24 hours. Failure to do so can lead to fines or legal action.
Is DSCSA the same as the EU’s FMD?
No. The EU’s Falsified Medicines Directive (FMD) requires physical anti-tampering seals and a central European database to verify drugs. DSCSA doesn’t use a central database. Instead, it relies on direct electronic communication between trading partners - manufacturers, distributors, and pharmacies - to verify each package. DSCSA focuses on data exchange; FMD focuses on physical safety features and a single national repository.
Will DSCSA cover over-the-counter drugs in the future?
Yes, possibly. In March 2023, FDA Commissioner Dr. Robert Califf said the agency is evaluating whether to extend DSCSA requirements to certain high-risk over-the-counter drugs - like insulin pens, epinephrine auto-injectors, or erectile dysfunction medications. These products are already targeted by counterfeiters, and extending the system could prevent harm before it starts.
What are the biggest challenges with DSCSA today?
The biggest issues are data mismatches between systems and inconsistent use of the EPCIS standard. Two pharmacies using different software might record the same serial number differently, causing false flags. Also, some vendors don’t follow EPCIS rules exactly, which breaks communication between systems. This causes delays in drug delivery and increases compliance risk. The FDA is working with industry to fix these interoperability problems.
How do I know if my medicine is safe under DSCSA?
You don’t need to check anything yourself. The system works behind the scenes. If your pharmacy can’t verify your drug - if the system says it’s suspect - they won’t give it to you. If you ever get a drug and it looks wrong - different color, smell, or shape - don’t take it. Return it and ask the pharmacist to verify it. That’s what the system is there for: to protect you when you can’t see the risks.




Mussin Machhour
December 24, 2025 AT 16:33 PMThis system is a game-changer. I work in a pharmacy and before DSCSA, we’d get shipments with mismatched lot numbers and just shrug it off. Now? We flag it, quarantine it, and sleep better at night. No more guessing if that pill is legit or not. It’s not perfect, but it’s the first real safety net we’ve ever had.
Jason Jasper
December 26, 2025 AT 08:07 AMHad a shipment get stuck for 48 hours last month because one system read the serial number in lowercase and the other didn’t. Frustrating as hell. But honestly? Better than handing out fake insulin.
Winni Victor
December 27, 2025 AT 22:51 PMOh please. This whole thing is just a cash grab for tech vendors. You think the FDA actually gives a damn about your grandma’s blood pressure meds? Nah. They’re just making sure Big Pharma doesn’t get sued when someone dies from a $2 pill that says ‘Lipitor’ but is just chalk and glitter. And don’t get me started on how much it costs small pharmacies. They’re getting crushed while Walgreens gets a tax break.
Sophie Stallkind
December 29, 2025 AT 04:33 AMWhile the operational challenges of implementing DSCSA are undeniably significant, particularly for independent dispensers, the ethical imperative to safeguard public health cannot be overstated. The systematic verification of pharmaceutical products constitutes a foundational advancement in patient safety protocol, and the transitional costs, however burdensome, are a necessary investment in the integrity of the medical supply chain.
Michael Dillon
December 29, 2025 AT 10:42 AMLet’s be real - 95% reduction in counterfeits? That’s PR spin. The real number is probably closer to 30%. You think bad actors can’t just clone serial numbers? They’ve been doing it for years. And the data mismatches? That’s not a glitch - that’s a feature. It’s designed to slow things down so the big guys can charge more for ‘compliance services.’
Oluwatosin Ayodele
December 30, 2025 AT 19:30 PMYou Americans think this is impressive? In Nigeria, we’ve been tracking drugs manually for decades with paper logs and WhatsApp groups. You spend millions on barcodes and still get delays. We just call the distributor and ask. No EPCIS. No software. Just trust and a phone call. Your system is over-engineered and fragile. Real-world systems don’t need 20-character serial numbers to stop fakes.
Carlos Narvaez
January 1, 2026 AT 19:17 PMIt’s not about safety. It’s about control. The FDA doesn’t want you to know how much of your medicine is sourced offshore. This system lets them audit everything - and quietly shut down anyone who questions the supply chain. You think this is for patients? It’s for liability.
Harbans Singh
January 2, 2026 AT 07:22 AMI’ve been a pharmacist for 22 years, and I’ve seen this whole system evolve. The tech is clunky, sure. But I’ve seen two patients walk out because their meds got flagged - and later found out the batch was contaminated. That’s not luck. That’s the system working. To the folks complaining about cost: I get it. But what’s the cost of a kid getting fake ADHD meds? I’d rather pay $185k than bury someone.
Justin James
January 3, 2026 AT 04:50 AMThink about this - what if the whole DSCSA system is a front? What if the serial numbers aren’t even being checked properly? What if the FDA’s database is just a hollow shell, and the real verification happens in some secret NSA server? I’ve talked to a guy who works for a software vendor - he said the ‘verification’ is just a ping to a server that sometimes doesn’t even respond. They just auto-approve if the barcode scans. That’s why there are so many false positives. It’s all smoke and mirrors. And the real counterfeiters? They’re already in the system - they helped build it. They own the software. They own the data. They own the compliance. You think you’re safe? You’re just a data point in a surveillance pyramid designed to make you feel secure while they profit from your fear.